Electricity Helps Chemistry: Electro-plating
A liquid which is decomposed when an electric current passes through it is called an electrolyte. The process is called electrolysis, and the two wires or plates dipping into the electrolyte are called electrodes. The electrode which is connected to the positive terminal of the cell or battery is called the anode. The electrode which is connected to the negative terminal of the battery is called the cathode.
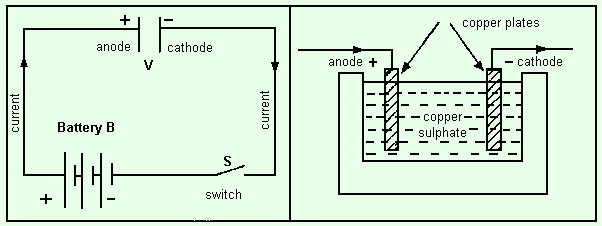
Let us examine what happens when two copper electrodes are used in a solution of copper sulphate. The circuit is shown in the diagram. The right-hand diagram shows the two copper electrodes dipping into the copper sulphate solution contained in a glass jar. The current enters by the anode (+), passes through the solution, enters the cathode (-), and then leaves the cathode as shown by the arrow. In the left-hand diagram, V represents the glass vessel containing the copper sulphate (electrolyte), and the two electrodes are marked + for the anode and - for the cathode. When the switch S is closed, the current flows from the - terminal of the battery B in the direction of the arrow to the anode (+) of V, through the solution to the cathode (-), then round the circuit through S back to the negative terminal of the battery B.
Before starting this experiment the weights of the two copper plates which are to be used for the anode and cathode must be written down carefully for future reference. Next, place the anode and cathode in the copper sulphate solution and connect them up to the battery B and switch S. The switch is then placed in the 'on' position and the current is allowed to flow through the circuit for about half an hour. The anode and cathode are then removed and dried carefully in blotting paper before being weighed a second time.
You will find that a surprising thing has happened. The anode now weighs a few milligrams less than before and the cathode weighs a few milligrams more than before. The weight lost by the anode is exactly equal to the gain in weight by the cathode. In some strange way a few milligrams of copper have been removed from the anode and carried through the electrolyte by the current and have finally become firmly attached to the cathode. This is a most exciting discovery, for we have learned how to use an electric current to transfer tiny particles of copper from the anode to the cathode.
Nineteenth-century industry soon found out how to apply this exciting discovery to our everyday lives. Scientists found that many other metals could be transferred from anode to cathode. The anode had to be made of the metal which it was desired to transfer to the cathode, and the electrolyte had to be a suitable solution or salt of the metal. Then the cathode always became plated with metal from the anode. Copper, silver, gold, nickel, zinc and chromium can all be used in this process, which is called electro-plating. Electro-plating is used widely in industry for a number of reasons. Firstly, it is used for decoration. Coatings of nickel, gold, silver or chromium give a nice shiny appearance to articles and make them look much more expensive. Watch-cases and cutlery are often plated with silver or gold to give them a smart appearance so that they become attractive to intending buyers. Handlebars of bicycles and the shiny fittings of cars are also made attractive by means of nickel and chromium plating.
This leads us to the second reason for electro-plating - as a protection against rust or corrosion. Iron and steel corrode easily when exposed to the atmosphere. Car fittings and the shiny parts of bicycles are electro-plated chiefly for this reason, so that they may stand up to the hard wear and tear of daily use. Zinc is formed into a protective layer for iron sheets by the electroplating process which we now call galvanizing. Galvanized iron sheets resist the effects of wind and weather much better than sheets made of iron. Tin is also used as a protective agent. Sheets of thin iron are plated with tin and used for canning fruit and jam, and for all kinds of 'tin' cans used in industry and trade. We may sum up by saying that industry has used the process of electro-plating first to protect metal surfaces which would otherwise corrode; and secondly to provide a beautiful and attractive finish to useful articles. As a result, our bicycles and cars, our watches and cutlery, our building and manufacturing materials last much longer and are much more pleasant to look at.
The process of electrolysis is used for the production of very pure specimens of metal. Most metals in industrial use contain many impurities. About 1 million tons of refined copper are produced each year by electrolysis. In this case the anode consists of crude copper and the cathode of thin sheets of pure copper. As the current passes, pure copper from the anode passes over to the cathode, and all impurities fall off the anode as a kind of mud. In this way pure copper is collected at one electrode and the muddy residue, which falls off the cathode, sinks to the bottom of the vat and is periodically removed.
Aluminium is so widely used today that we can scarcely think of times when it was not available. Yet a few years back it was a costly metal because no satisfactory method had been found of producing it commercially. Aluminium ores are so common in nature that scientists and engineers made many attempts to find a cheap and convenient method of refining them. The problem was finally solved by electrolysis, using a carbon anode and aluminium ores, which had been melted at a temperature of about 1,000�C, as the electrolyte. Aluminium is now plentiful and it is being put to fresh uses every day.
Electrolysis has an important industrial application in the printing trade, for it is often used to make the 'blocks' from which pictures and type are printed. A wax mould is first made of the printing block which is to be reproduced. Since wax is a non-conductor of electricity it is dusted over with graphite so that the surface becomes a conductor and can act as a cathode. This mould then becomes the cathode upon which copper or chromium is deposited from the anode. When the wax is taken out of the electrolyte it is coated with a fine shell of metal. The wax is removed by heating and the metal shell acts as a mould into which molten type metal can be poured. Plates made in this way are very hard-wearing and can be used to print many thousands of copies of newspapers, journals and magazines.
(From General Science by N. Ahmad, W. F. Hawkins And W. M. Zaki.)